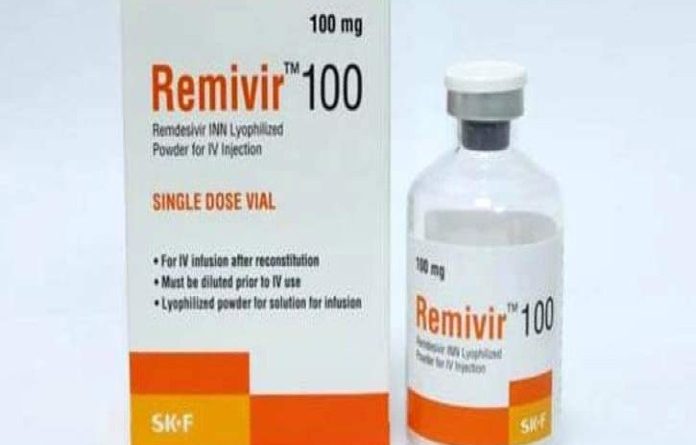
Bangladesh produces world’s first generic Remdesivir for Covid-19 treatment
Bangladesh has produced Remdesivir, a drug which is recognised as very effective for the treatment of coronavirus.
With that, Bangladesh becomes first in the world to sell generic version of remdesivir to treat Covid-19.
Remdesivir is an injectable drug.
According to Media reports, Bangladesh can produce generic versions of patented drugs under World Trade Organization provisions that grant least developed countries a waiver from seeking licenses.
Eskayef Pharmaceuticals
Directorate General of Drug Administration (DGDA) has said the drug will be delivered to hospitals after its final performance test.
According to rules, the samples will be submitted to the Bangladesh National Control Laboratory for medicinal products (NCL) under the DGDA, and distribution will start as soon as clearance and marketing permission are obtained, SKF said.
The company also said they made a deal with the main producer of the drug and US company Gilead Sciences Inc, and they have adequate raw materials for producing the drug.
SKF’s Director of Marketing and Sales Dr. Mujahidul Islam said they have completed all the steps of manufacturing the world’s first Remdesivir. “We’ll be able to deliver the drugs to the hospital just on completion of a process.
The DGDA said they will allow the use of the drug after its final performance test.
Major General Mahbubur Rahman, Director General of the DGDA, said the government and the DGDA have been working together to bring the drug in the market.
“It’s revolutionary incident that Bangladesh is producing such a drug.”
In addition to SKF, the DGDA has allowed eight other pharmaceutical companies, including Beximco, Beacon Incepta and Square Pharmaceutical companies to produce the drug.
Beximco is branding the drug bemsivir, the launch of which was announced during a simple ceremony at the Health Ministry in the presence of Zahid Maleque MP, Minister for Health and Family Welfare, Government of the People’s Republic of Bangladesh.
Product of BEXIMCO is already approved on 21 May 2020, that of SKF and INCEPTA would be approved on 24 May and 2 June 2020 respectively.

While handing over the first batch of medicine, Managing Director of Beximco Pharma, Nazmul Hassan MP, said: “We have decided to provide bemsivir free of cost to all those severely ill patients of government hospitals.”
U.S. drug authorities granted emergency use authorisation at the end of April, paving the way for its broader use in U.S. hospitals, after Gilead provided data showing the drug had helped Covid-19 patients.
Beximco says the drug can be given via intravenous infusion. Beximco’s Chief Operating Officer Rabbur Reza told Reuters that a patient might need anywhere between 5 and 11 vials.
The Japan government has also granted special approval for remdesivir in emergency treatment of Covid-19.
The emergency approvals will help broaden use of remdesivir in hospitalised patients, especially in developing and less-developed countries where access to breakthrough, advanced drugs remains a major challenge.
“We are pleased to be the first generic company in the world to introduce this very important drug for treating the hospitalised Covid-19 patients. This reinforces our commitment to playing our part in ensuring access to breakthrough therapies despite facing many challenges amid this unprecedented pandemic.
“We express our gratitude to the regulatory authorities for extending their wholehearted support for making this potential drug available to our patients at the earliest possible time. As a responsible company, we will continue to extend our support to the government in all possible ways during this national emergency,” Hassan said.
Zahid Maleque MP, Minister for Health and Family Welfare, Government of the People’s Republic of Bangladesh, lauded Beximco Pharma for its initiatives during this time of the pandemic.
He said: “Beximco Pharma has taken various proactive measures to provide the latest, evidence-based treatment options for Covid-19 patients. The company is committed to fighting this unprecedented pandemic, in all possible ways, employing its competitive R&D and manufacturing skills.”
Beximco Pharma which has geographic footprint in more than 50 countries has already launched another anti-viral drug, favipiravir, under the brand name viraflu, as well as re-purposed anti-malarial drug, hydroxychlorqui
Nigeria may perhaps benefit immensely from the product once formalities for import are done.